Earth-Abundant Molecular Catalyst Systems for the Reduction of Dioxygen and Carbon Dioxide
Charles Machan, University of Virginia
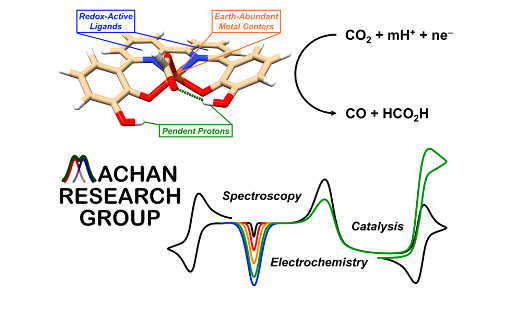
The steady increase in anthropogenic carbon dioxide (CO2) emissions and corresponding atmospheric concentrations continues to generate interest in using CO2 as a liquid fuel and commodity chemical precursor. The conversion of CO2 has the dual benefit of addressing its associated negative environmental effects and the diminishing supply of petrochemical feedstocks. At the heart of efficient reductive transformations are proton-coupled electron transfer (PCET) reactions, where electrons and protons move in a concerted way to mitigate kinetic and thermodynamic penalties. Mechanistic understanding of these reactions can inform the design of optimized catalyst structures with improved activity and selectivity for specific products. Another cathodic reaction, the electrocatalytic reduction of dioxygen (O2), has relevance to the development of more efficient fuel cells and can be similarly optimized through fundamental knowledge of the underlying PCET reactions. Molecular systems are well-positioned to provide a better understanding of these reactions because of the relative fidelity with which they can be characterized, as well as the possibility for systematic testing of structure-function relationships through iterative molecular design. In addition to developing new Fe-, Mn-, and Cr-based molecular electrocatalysts for these reactions, we are exploring the use of redox mediators and flow-based electrochemical reactors to understand how these reactions can be scaled relative to comparable heterogeneous systems.
Host: Bill Tolman