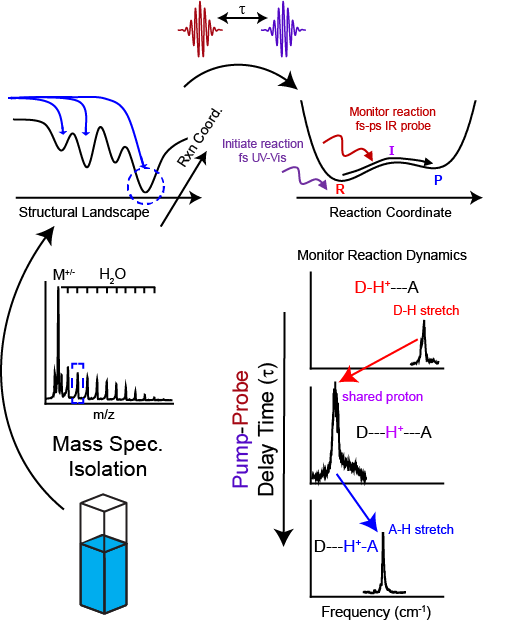
The Fournier lab combines the sensitivity and selectivity of mass spectrometry with the time resolution of ultrafast spectroscopies to capture and directly interrogate fast chemical reactions with molecular-level detail.
Identification and characterization of transient intermediates is crucial for developing detailed molecular-level knowledge of chemical reaction mechanisms – insight that is required to fully understand important biological processes like photosynthesis, energy storage and transfer, and for the rational development of novel synthetic catalysts. However, key reaction intermediates and their dynamics are often too short-lived or technically challenging to capture and interrogate directly with current methods. Next‑generation methods are required to isolate and probe elusive reaction intermediates and transition‑state species with atomistic detail.
Professor Fournier's research program seeks to develop novel experimental techniques which will allow for the capture and direct interrogation of reaction intermediates by combining the high signal sensitivity of mass spectrometry, the high frequency resolution of gas‑phase ion spectroscopies, and the time resolution of ultrafast spectroscopies in a single experiment. The versatility of mass spectrometric techniques allows for the careful control and manipulation of the chemical environment around the reactive system of interest in a composition-selective manner. The well resolved optical spectra that will be obtained from the isolated and cryogenically cooled (10 K) ensembles will yield unambiguous structural identification, while the time evolution of the spectroscopic transitions with ultrafast resolution will characterize the time-evolving shape of the reactive potential energy surface (PES). This data will provide the basis for clear mechanistic interpretation of how the surrounding chemical environment actively dictates the observed reaction dynamics and underlying shape of the PES.
Specifically, he is interested in studying catalytic processes driven by proton-coupled electron transfer (PCET), which are ubiquitous throughout chemistry and biology. His focus includes two forefront problems where clear mechanistic details are vitally needed: (1) The role of tyrosine and tryptophan in biological PCET, in particular, the nature and dynamics of TyrOH•+ and TrpNH•+ radical cation species which are proposed key intermediates in systems including Photosystem II, ribonucleotide reductase, and cytochrome c oxidase. (2) Capturing intermediates generated during the activation of small molecules by organometallic catalysts, specifically, how solvent waters around the active site and ligand composition in water oxidation catalysts drive the formation of the proposed high-valent metal-oxo intermediate.