The Wexler Group focuses on theoretical materials innovation for renewable energy and environmental applications. We develop advanced computational methods to model interfacial phenomena in electrocatalysis, solar energy conversion, and environmental energy harvesting. Using first-principles calculations, Monte Carlo simulations, and machine learning, we aim to understand complex materials systems, discover structure-function relationships, identify promising materials, and optimize them for enhanced performance.
Predicting Equilibrium Structures of Catalyst Surfaces
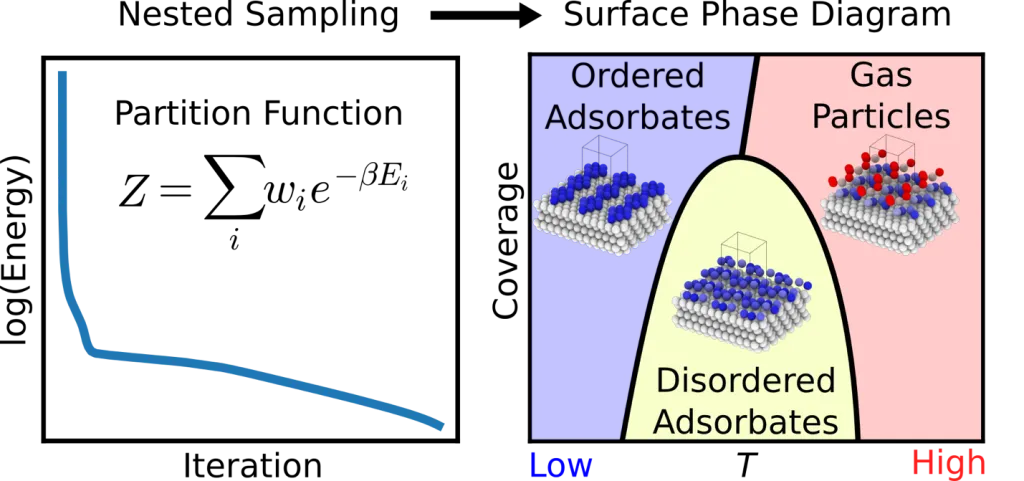
Heterogeneous catalysts are essential for industrial chemical production. Enhancing their efficiency requires an atomic-level understanding of surface structures and active sites. Key factors in catalyst design include surface reconstruction and degradation. Surface reconstruction involves changes in periodicity, species coordination, composition, and thickness along the surface normal, differing from the bulk material. Degradation refers to the gradual loss of catalytic activity and selectivity over time due to environmental or operational stresses. The operating environment significantly impacts these transformations, affecting catalyst activity and selectivity. Designing efficient catalysts necessitates understanding the conditions under which surfaces restructure in situ. Surface structure and composition measurements are often complicated by interactions with solutes and solvents, ex-situ oxidation, and reactive conditions that can damage experimental apparatus. This project focuses on developing computational methodologies to predict the equilibrium reconstructions of catalyst surfaces. Although active catalysts are typically out of equilibrium, they can establish a surface equilibrium before catalysis begins. The primary goal is to identify the most stable catalyst surface form, providing a foundation for further studies on its behavior during active catalysis. As a proof-of-concept, we applied nested sampling to the adsorption of Lennard-Jones gas particles on low-index and vicinal Lennard-Jones solid surfaces. By constructing the canonical partition function from the recorded energies, we can calculate ensemble averages of thermodynamic properties, such as constant-volume heat capacity and order parameters that characterize adsorbate phase structures.
Ca-Ce-Ti-Mn-O-Based Perovskites for Solar Thermochemical Hydrogen Production
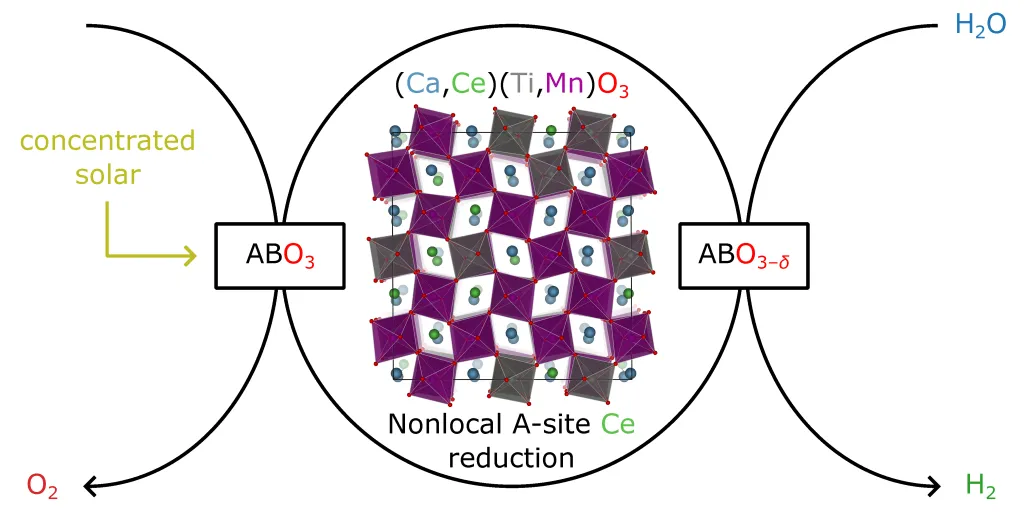
Two-step solar thermochemical hydrogen production (STCH) cycles utilize redox-active metal oxides (MOx) that undergo thermal reduction and re-oxidation to split water and produce hydrogen. The STCH cycle involves heating MOx to temperatures exceeding 1500 K using concentrated solar radiation. The material reduces to an oxygen-deficient state (MOx–δ) at these high temperatures and low oxygen partial pressures. In the subsequent step, the reduced MOx is exposed to superheated steam, leading to re-oxidation and water splitting, generating hydrogen and regenerating the original MOx. Our perovskite material, (Ca, Ce)(Ti, Mn)O3–δ, exhibits remarkable off-stoichiometric redox activity, forming and filling oxygen vacancies during the thermal reduction and re-oxidation steps without significant bulk phase transitions, enhancing kinetics, cyclability, and durability. This interdisciplinary project integrates theoretical modeling, synthesis, characterization, material thermodynamics, reactor design and prototyping, system mass/energy flow analysis, and technoeconomic analysis. The goal is to develop a robust, cost-effective STCH cycle using (Ca, Ce)(Ti, Mn)O3–δ perovskites, leveraging their compositional flexibility and abundant metal precursors. Building on HydroGEN advances, this research addresses affordability, stability, and large-scale clean hydrogen production challenges.
Thermochemistry and Kinetics of Halide-driven Crystal Structure Control in Mn and Ln Chalcogenide Nanocrystals
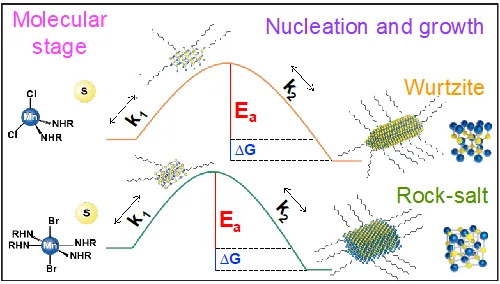
This research aims to develop a framework combining experimental and computational methods to understand how halides influence the crystal structure and phase of manganese chalcogenide nanocrystals during synthesis. Key objectives include identifying pre-nucleation species, conducting thermochemical measurements of reactions and surface-ligand interactions, and monitoring nucleation and growth kinetics using in situ techniques. We use quantum-mechanics-based calculations to reveal atomic-scale interactions and mechanisms determining crystal structures and phases. These calculations inform kinetic and thermodynamic models of nanocrystal nucleation and growth, providing multi-scale computational guidance for controlled synthesis. The research extends to lanthanide chalcogenide nanocrystals, which have unique optical and magnetic properties but are less explored. The ultimate goal is to enhance the chemical understanding of Mn and Ln chalcogenide nanocrystal synthesis, offering insights for the broader scientific community and aiding in synthesizing other material classes.


