Our group’s research efforts are in the area of synthetic organic chemistry. Over the years, we have focused on total synthesis of bioactive natural products, asymmetric catalysis, stimuli-responsive molecules and, most recently, fluorescent dyes for bioimaging applications. In summary, we are interested both in devising synthetic strategies towards targets defined by Nature and in rationally designing molecules with a defined function.
TOTAL SYNTHESIS. In 2004, we completed total syntheses of the unusual marine alkaloid sceptrin.[1] In 2007, we disclosed a regioselective synthesis of the antibiotic prekinamycin,[2] utilizing a novel annulation reaction and an enantioselective synthesis of the alkaloid lobeline[3] via the desymmetrization of a meso-diol precursor using one of our catalysts, BTM (see below). In 2016, we achieved a concise enantioselective synthesis of the meroterpenoid lingzhiol[4] featuring a biogenetically-inspired semipinacol rearrangement (Figure 1).
Figure 1. Completed synthetic targets


ASYMMETRIC CATALYSIS. Our efforts in the area of asymmetric catalysis have concentrated on enantioselective acyl transfer. Our group has developed a new class of enantioselective acyl transfer catalysts (ABCs, for Amidine-Based Catalysts, Figure 2).[5] These compounds, easily obtained from commercially available starting materials, are highly effective in the kinetic resolution (KR) of several classes of chiral secondary alcohols (Figure 3a).[6] In addition, we have demonstrated for the first time the KR of chiral lactams and thiolactams via catalytic, enantioselective N-acylation (Figure 3b).[7] The enantioselectivities observed in all these cases are consistent with a transition state model based on cation-p interactions between the acylated intermediate and the substrate. Our computational studies in collaboration with the Houk group (UCLA) support this hypothesis.[8] Most recently, we also achieved KR of cyclic hydroxamic acids via O-acylation (Figure 3c).[9] ABCs have also proved effective in promoting enantioselective alcoholysis of several classes of racemic acyl donors (Figure 4).[10] Some of these reactions proceed in the dynamic kinetic resolution (DKR) mode converting both enantiomers of the starting material into a signle enantiomer of the product.
Figure 2. Evolution of amidine-based catalysts (ABCs)


Figure 3. Enantioselective catalytic acylation promoted by ABCs


Figure 4. Enantioselective catalytic alcoholysis promoted by ABCs


A different type of applications in which ABCs have demonstrated their unique efficacy is cascade reactions accompanied by generation of new stereocenters. For example, we have disclosed a highly enantioselective transformation of thioesters into chiral thiochromenes requiring no additional reagents and giving off carbon dioxide as the only byproduct (Figure 5a).[11]a Interestingly, a closely related rearrangement of thioesters into tricyclic thiochromanes (Figure 5b) proceeded very slowly with HBTM-2. By contrast, the electron-rich catalyst H-PIP (shown) displayed excellent rates and enantioselectivities in this reaction.10b
Figure 5. Enantioselective rearrangement of thioesters promoted by ABCs


Another useful application of ABCs that we have developed is the enantio- and diastereo-selective synthesis of α-fluoro-β-amino acid derivatives by way of fluorinated β-lactams (Figure 6).[12]
Figure 6. Synthesis of α-fluoro-β-amino acid esters


REDOX-RESPONSE MOLECULAR SWITCHES AND OLIGOMERS. Ten years ago, we became interested in the de novo design of “molecular muscles”. In this context, we envisioned phenazine-1,6-dicarboxamides functioning as double molecular switches unfolding upon reduction and folding back upon re-oxidation (Figure 7a). A chain composed of such molecular switches would extend and contract reversibly in response to reduction-oxidation thus mimicking the function of actin-myosin filaments in Nature (Figure 7b). In 2020, we published our first proof-of-principle study (Figure 8).[13] In 2022, we demonstrated that phenazine-based oligomers such as those shown in Figure 9 undergo fully reversible extension-contraction in response to chemical or electrochemical stimulation.[14]
Figure 7. Phenazine-based molecular switch: the concept

Figure 8. Proof-of-principle study.

Figure 9. Redox-responsive phenazine-based oligomers


For more details and other projects pursued in our group, please see additional references provided in the CV.
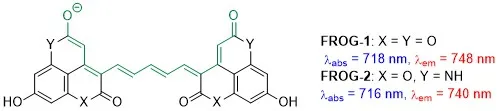
NEAR INFRARED FLUOROPHORES. Fluorescent dyes are widely used in a variety of optical bioimaging applications. Those that absorb and emit light in the near-infrared (NIR) part of the spectrum (700-900 and 1,100-2,000 nm) are of particular interest because biological tissues are transparent in that range, which enables deeper penetration and better resolution. In collaboration with the group of Prof. Mikhail Berezin (Department of Radiology, Wash. U. School of Medicine), we have recently devised a promising new class of NIR-active fluorophores dubbed FROGs, which stands for Fiercely Radiant Oxonol Green (Figure 10).[15] Our current efforts are focused on the development of this and other new classes of fluorophores and their applications in bioimaging.
Figure 10. FROGs: novel NIR fluorophores
For more details and other projects pursued in our group, please see additional references provided in the CV.
[1] Birman, V. B.; Jiang, X.-T. Org. Lett. 2004, 6, 2369. DOI: 10.1021/ol049283g
[2] Birman, V. B.; Zhao, Z.; Guo, L. Org. Lett. 2007, 9, 1223. DOI: 10.1021/ol0629768
[3] Birman, V. B.; Jiang, H.; Li, X. Org. Lett. 2007, 9, 3237. DOI: 10.1021/ol071064i
[4] Sharmah Gautam, K.; Birman, V. B. Org. Lett. 2016, 18, 1499. DOI: 10.1021/acs.orglett.5b03212
[5] For a review, see: Birman, V. B. Aldrichimica Acta 2016, 49, 23.
[6] Li, X.; Jiang, H.; Uffman, E. W. Guo, L.; Zhang, Y.; Yang, X.; Birman, V. B. J. Org. Chem. 2012, 77, 1722. DOI: 10.1021/jo202220x and references cited therein.
[7] Yang, X.; Bumbu, V. D.; Liu, P.; Li, X.; Jiang, H.; Uffman, E. W. Guo, L.; Zhang, W.; Jiang, X.; Houk, K. N.; Birman, V. B. J. Am. Chem. Soc., 2012, 134, 17605. DOI: 10.1021/ja306766n and references cited therein.
[8] Li, X.; Liu, P.; Houk, K. N.; Birman, V. B. J. Am. Chem. Soc. 2008, 130, 13836. DOI: 10.1021/ja805275s
[9] Yin, J.; Straub, M. R.; Liao, J.; Birman, V. B., Org. Lett. 2022, 24, 1546-1549.
[10] (a) Yang, X.; Birman, V. B. Chem. Eur. J. 2011, 17, 11296. DOI: 10.1002/chem.201101028 and references cited therein. (b) Yang, X.; Lu, G.; Birman, V. B. Org. Lett. 2010, 12, 892. DOI: 10.1021/ol902969j (c) Bumbu, V. D.; Birman, V. B. J. Am. Chem. Soc. 2011, 133, 13902. DOI: 10.1021/ja2058633 (d) Bumbu, V. D.; Yang, X.; Birman, V. B. Org. Lett. 2013, 15, 2790. DOI: 10.1021/ol401122g
[11](a) Ahlemeyer, N. A.; Birman, V. B. Org. Lett. 2016, 18, 3454 DOI: 10.1021/acs.orglett.6b01639 (b) Ahlemeyer, N. A.; Streff, E. V.; Muthupandi, P.; Birman, V. B. Org. Lett. 2017, 19, 6486. DOI:10.1021/acs.orglett.7b03044
[12]Straub, M. R.; Birman, V. B. Org. Lett. 2018, 20, 7550 DOI:10.1021/acs.orglett.8b03297
[13] Yin, J.; Khalilov, A. N.; Muthupandi, P.; Ladd, R.; Birman, V. B., J. Am. Chem. Soc. 2020, 142, 60-63. DOI: 10.1021/jacs.9b11160
[14] (a) Yin, J.; Birman, V. B. Org. Lett. 2022, 24, 8759-8763. DOI: 10.1021/acs.orglett.2c03450 (b) Yin, J.; Birman, V. B. J. Org. Chem. 2022, 87, 15744-15753. DOI: 10.1021/acs.joc.2c01445
[15] Fuentes, I.; Tang, R.; Michie, M.; Lin, C.; Berezin, M.;* Birman, V. B.* Near Infrared fluorescent oxanol dyes with 1,6-diheterophenalene-2,5-dione end groups, Chem. Biomed. Imaging 2025, ASAP. https://pubs.acs.org/doi/10.1021/cbmi.5c00205

