The Okuno research group aims to develop both the theoretical and experimental methods needed to understand the chemicophysical nature of ligand-protein and protein-protein interactions at the molecular level.
We put emphasis on understanding the roles of solvent (e.g., water) and weak noncovalent interactions such as NH-π and CH-π interactions in ligand-protein and protein-protein binding processes. Nuclear Magnetic Resonance Spectroscopy (NMR) is a powerful technique that provides atomic-level information about proteins and protein interactions. We use NMR spectroscopy as well as other experimental and computational approaches to unravel challenging biological processes.
Current projects in the lab include:
The role of water in protein-ligand association Water is the most abundant molecule in any biological system. Elucidating the molecular details of how water molecules interact with proteins is therefore critical for understanding any types of biological processes.
By taking advantage of the fact that water molecules generate weak magnetic fields, we investigate how water molecules interact with a protein at the atomic level. For example, NMR observable known as the nuclear Overhauser effect (NOE) provides rich information about both the dynamic and energetic of water-protein interactions. We aim to develop new experimental methods to detect weak water-NOE signals and develop a theoretical basis to rigorously interpret the experiments.
The weak noncovalent protein-ligand interactions in IDP Many drugs and other small molecule ligands utilize weak noncovalent interactions to bind to their targets. For example, many drugs consist of aromatic groups capable of NH-π and/or CH-π interactions with a protein surface.
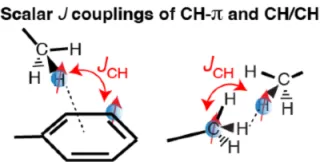
NMR spectroscopy enables direct detections of NH-π, CH-π, and CH-CH interactions at atomic resolution by so-called “through-space” J-coupling. The J-coupling can be detected only when there is an electron cloud between the two nuclei, thereby providing the direct evidence of the existence of hydrogen bonds. We aim to develop new experimental methods to measure through-space J-coupling tailored to probe ligand-protein interactions.
Probing the molecular forces in protein aggregation and in liquid-liquid phase separation The amyloid beta peptides known as Aβ(1-42) and Aβ(1-40) rapidly form aggregation species and the aggregates are known to be involved in Alzheimer’s disease. Many drugs are targeted to prevent these aggregation pathways of Aβ peptides. However, not much is known about the mechanisms of how Aβ peptides form these aggregates at the molecular level. Using the NMR as well as other experimental techniques, we explore what chemical forces drive the early stage of Aβ peptides aggregation processes with the ultimate goal to develop a new therapy for Alzheimer’s diseases and other neurodegenerative diseases.
Selected publications
Okuno, Y., Schwieters, C.D., Yang, Z., Clore, G.M. Theory and Applications of Nitroxide-based Paramagnetic Cosolutes for Probing Intermolecular and Electrostatic Interactions on Protein Surfaces. J. Am. Chem. Soc, 144 (46), 21371-21388
Okuno, Y., Yoo, J., Schwieters, C.D., Best, R.B., Chung, H.S. & Clore, G.M. Atomic view of cosolute-induced protein denaturation probed by NMR solvent paramagnetic relaxation enhancement. Proc. Natl. Acad. Sci. 118, e211202118
Okuno, Y., Szabo, A., Clore, G. M. Quantitative interpretation of solvent paramagnetic relaxation for probing protein–cosolute interactions. J. Am. Chem. Soc. 2020, 142 (18) 8181-8290
Okuno, Y., Mecha, M. F., Cavagnero, S. Laser-and cryogenic probe-assisted NMR enables hypersensitive analysis of biomolecules at submicromolar concentration, Proc. Natl. Acad. Sci. 116 (24), 11602-11611
Okuno. Y., Cavagnero, S.,Effect of heavy atoms on photochemically induced dynamic nuclear polarization in liquids, J. Magn. Reson. 2018, 286, 172-187
