Researchers in chemistry develop new and improved method for synthesizing chains of mechanically interlocked molecules.

Most molecules are held together by chemical bonds – you might recall covalent and ionic bonds from chemistry class – but mechanically interlocked molecules (MIMs) rely on their mechanical structure or topology to keep components together. Because they aren’t bonded in the traditional sense, MIMs offer an increased range of motion. They can stretch, compress, rotate, and in theory, absorb energy more effectively, making them attractive additions to hydrogels and other functional polymeric materials.
New research from Jonathan Barnes’ lab in the Department of Chemistry introduces a streamlined method for synthesizing chains of MIMs. This foundational work develops a novel way of creating linear chains with four rings in one step – a feat that has previously been accomplished less than a handful of times – and sets the stage for scaling up in future work. The research is published in the journal Inorganic Chemistry.
In their new work, Jonathan Barnes, assistant professor of chemistry, lead author Nathan Colley, and co-author Mark Nosiglia, graduate students in Barnes’ lab, have focused on increasing the efficiency and yield of MIM synthesis.
“The key feature of MIMs is that the molecules can’t be torn apart without breaking covalent bonds, but they aren’t actually covalently linked,” said Nosiglia. “Traditional polymer chains work like a rope or a cord. When you pull them, there’s no slack between molecules, so they can only move in limited ways. Whereas when we put rings together in a chain, there’s extra space for the rings to move around each other.”
The resulting molecules retain the strength of covalent bonds while also gaining freedom of movement. Nosiglia also pointed to increased flexibility and durability as features of bulk materials made from these linear chains or catenates.
Though mechanically interlocked molecules have many advantageous properties, synthesizing molecules with mechanical bonds is remarkably difficult. Nontraditional bonds require innovative and carefully tailored solutions.
“It’s incredibly challenging and only produces small quantities,” Barnes said. “We’re able to produce milligrams right now, not the grams or kilograms required to make a bulk material that could have an application like impact resistance or something like that. So there’s a big gap between really cool fundamental chemistry and what we want to do with it.”
Though Barnes’ team only produced about 50 milligrams of material in this set of experiments, the efficiency and high percent yields they were able to achieve are promising.
Expanding on earlier methods for forming molecular chains or linear catenates, the Barnes Lab was able to bring together two open molecular rings with two closed ones in a stable configuration. Then they used a ring closing reaction to create their desired four-ring catenate (see figure below). This carefully orchestrated synthesis results in three mechanical bonds between four rings in only one process, eliminating the need for intermediate purification of the resulting products.
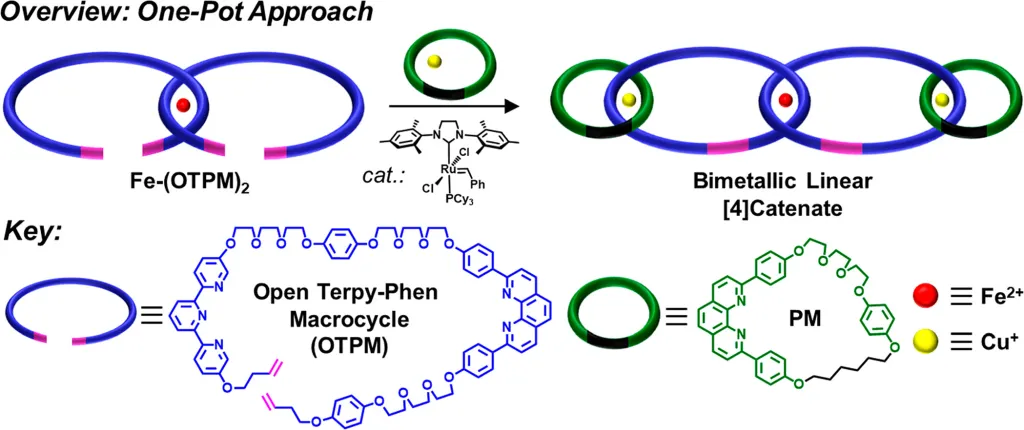
The foundational improvements in the process increase yield and open the door for longer chains.
“Linear polycatenanes, long chains which possess the highest concentration of mechanical bonds, are still a formidable target in the field of polymer science,” said lead author Nate Colley. “One of the major challenges in the synthesis of linear polycatenanes is the lack of control with most template strategies prior to ring closing, which leads to a mixture of linear and branched species. In order to improve template control, we employed an orthogonal approach that utilizes multiple templates at once for the efficient synthesis of a linear [4]catenate. By providing enhanced template control, we envision that this orthogonal templation strategy will serve as a general blueprint for the synthesis of linear polycatenates.”
“That’s the point of this work. We didn’t invent a new way to make mechanical bonds, but we did take a huge step forward by figuring out a way to form multiple mechanical bonds at the same time,” Barnes continued. “Making three mechanical bonds in one reaction is not trivial. Our next steps will be to figure out how to make this more efficiently so we can make the chain longer, and then make enough of that material to test macro-scale properties like impact resistance and force dissipation.”
“This is just the beginning,” Barnes added.
Acknowledgements
This work was supported by the David and Lucile Packard Foundation in the form of Jonathan Barnes’ Packard Fellowship for Science and Engineering.



