D’Arcy Lab develops an elegant, inexpensive approach that uses aerosolized water vapor as a template for creating uniform plastic nanoparticles.
Current methods for producing conducting plastics typically deposit the material onto a flat surface, resulting in differences in shape between the top and bottom of the particles. The trick, according to Julio D’Arcy, assistant professor of chemistry, is figuring out how to make particles homogeneous.

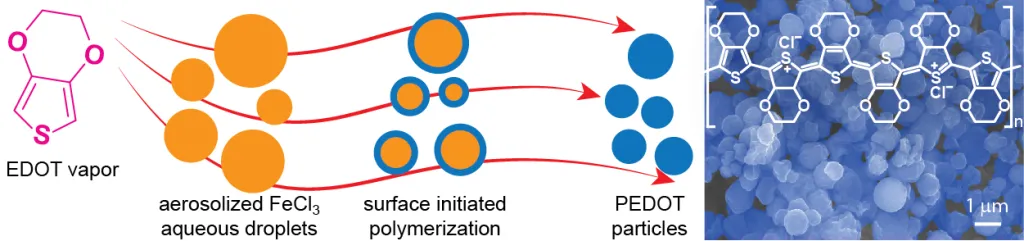
To produce perfect particles, D’Arcy and his team turned to an unlikely source: drugstore humidifiers. “Common humidifiers, like what you might buy at Walgreens, are incredible little devices,” D’Arcy said. “They produce droplets that are 1-3 microns in size, and they only cost about $20. So we thought, why don’t we start with these 3 micron sized droplets for our syntheses?”
D’Arcy and his collaborators from the Department of Chemistry and Washington University’s Institute of Materials Science and Engineering (IMSE) published their research, titled “Synthesis of Submicron PEDOT Particles of High Electrical Conductivity via Continuous Aerosol Vapor Polymerization,” on November 19 in ACS Applied Materials & Interfaces.
D’Arcy started this project with the goal of controlling the shape of synthesized conducting plastics at the nanoscale. Precise control is required for cutting-edge applications in semiconductors and energy storage, areas where D’Arcy has already made advances in the durability of materials.
Lead author Yang Lu, a graduate student in D’Arcy’s group who is also affiliated with IMSE, incorporated hardware from a simple humidifier into a fume hood. This apparatus can make an aerosol out of droplets of water. Depositing their base material on aerosolized water vapor allowed the team to create the desired homogeneous particles.
“We took the chemistry out of making nanoparticles,” D’Arcy said. “Normally, whenever chemists make a material, we’re very concerned about controlling the interactions of the components – how they mix and form new materials at the molecular scale. Here, we don’t need the materials to self-assemble at the molecular scale. Instead, we control the nanoscience with an inexpensive vaporizer.”
Their novel method starts with small aerosolized droplets that are made even smaller by heating them to evaporate some of the water. These tiny droplets then serve as templates for nanoparticles. The template droplets aren’t pure water. They contain a salt that reacts with a vaporized monomer, causing the material to stick to the surface of the droplets to form conducting polymer particles.
Not only are the resulting particles homogeneous in shape, they also show better conductivity and stability than any other currently available conducting plastic particle. These are essential properties for use in supercapacitors and batteries.
“Our particles are not like others out there,” D’Arcy said. “These organic semiconductors are physically and chemically stable for months, and, to our knowledge, have the highest reported electrical conductivity of any solid-state conducting polymer particle. The particles can withstand heat and changes in electrical potential while remaining functional.”

D’Arcy’s synthesizing process allows polymer particles to be sorted by size and collected as either a liquid dispersion or a powder. Producing the material in different forms and sizes results in increased versatility, with applications across fields ranging from thermoplastic composites for 3-D printing to sulfur concrete used in specialized construction projects. Because the semiconducting polymer is sensitive to temperature and PH, which are indicated by changes in the polymer’s electrical potential and color, it offers increased functionality in an array of materials and applications.
“We’re not just focused on delivering particles,” D’Arcy said. “We’re focused on delivering particles with the right properties, and we’re focused on delivering a product that’s stable. That’s possible because my lab’s expertise has always been about how bonds assemble. We have closed the gap on particle synthesis, achieving high chemical and physical stability, which was not in the market.”
The new, robust synthesis method is just a start. D’Arcy’s team is already looking for ways to create even smaller particles that might find uses in electrodes, semiconducting devices, or coatings for biological applications. The team will also need to significantly increase the rate of particle production before the material sees extensive commercial applications.
Ongoing collaborations across campus will be essential going forward, D’Arcy noted. To develop a future scalable technology, the D’Arcy laboratory will work along with Pratim Biswas, chair of the Department of Energy, Environmental & Chemical Engineering in the McKelvey School of Engineering. Biswas is an expert in aerosols, as well as a co-author and key collaborator in the continuing development and expansion of this new aerosol vapor polymerization method. To probe the structure of the polymer itself, this materials chemistry group will continue collaborating with Jacob Schaefer, Charles Allen Thomas Emeritus Professor of Chemistry, another co-author and a leader in solid-state NMR. Schaefer’s NMR techniques are uniquely well suited for studying solid-state conducting materials like polymer particles.



